

The (n+l) rule can be used to establish the sequence in which the energy of orbitals grows, with the sum of the primary and azimuthal quantum numbers determining the orbital’s energy level.This means that electrons can only enter higher-energy orbitals after lower-energy orbitals have been entirely filled. The Aufbau principle states that electrons will inhabit the lowest-energy orbitals first.
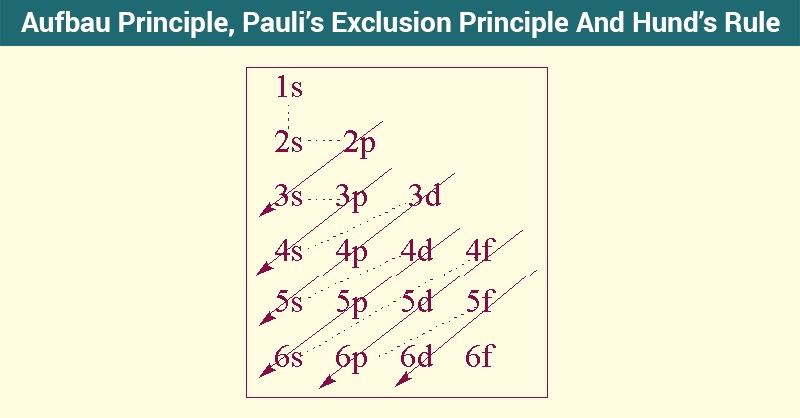
In addition, the way electrons are filled into orbitals in a single subshell must adhere to Hund’s rule, which states that every orbital in a given subshell must be single-occupied by electrons before any two electrons pair up in an orbital. Carbon, for example, has six electrons and has the electrical structure 1s22s22p2.Īufbau Principle is worth noting that each orbital can only store two electrons (according to Pauli’s exclusion principle). The Aufbau principle can be used to figure out where electrons are in an atom and what energy levels they correspond to. The phosphorus atom, for Aufbau Principle example, has the configuration 1s2 2s2 2p6 3s2 3p3, indicating that the 1s subshell possesses two electrons, and so on. An atom’s or ion’s electrons form the most stable electron configuration feasible in this fashion. According to Aufbau Principle The 1s subshell, for example, is occupied before the 2s subshell. The Aufbau principle, sometimes known as the Aufbau rule, asserts that when an atom or ion is in its ground state, electrons occupy subshells with the lowest possible energy first, then subshells with higher energy. Aufbau Principle Rule- The Ground State of Multi-electron Atoms/Ions.NCERT Solutions Class 10 Social Science.NCERT Solutions For Statistics Class 11.


 0 kommentar(er)
0 kommentar(er)
